About TrialSight
Our methodology has been clinically tested and shown to be effective1,2.
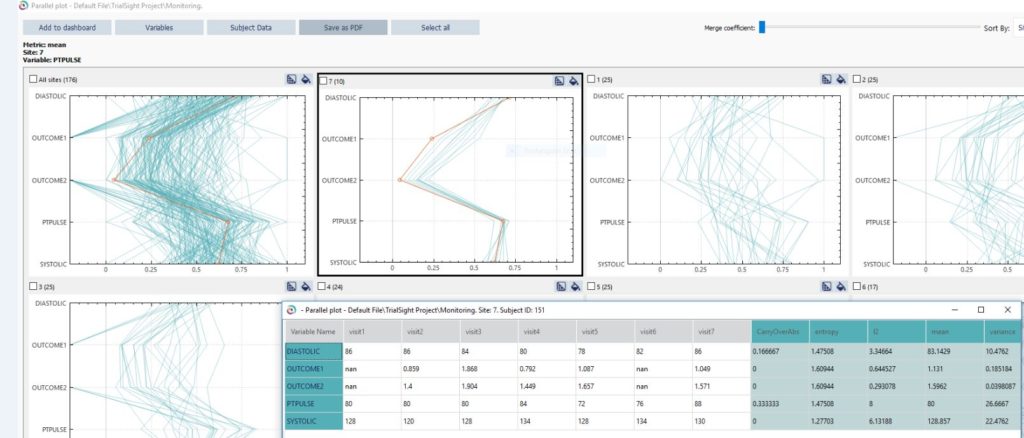
Central Statistical Monitoring applies statistical methodology to the holistic and continuous review of operations and clinical data at the study participant, site, examiner, region, study and program level. This software includes within-site examination of expected distributions of variables, imbalances in treatment assignment, patterns of variability, digit preference, weekend frequency of visits or testing, and carryover. The automated tool set evaluates:
- Modeling and pattern detection
- Visual analytics
- Quantification of signal vs. noise
- Continuous integration of algorithms and knowledge from on-site or off-site monitoring
The TrialSight Advantage
TrialSight is an integral part of risk-based monitoring and is being used extensively on trials funded by the National Institutes of Health, Foundations, and Industry. Findings from TrialSight have quickly identified retraining needs early in a trial’s execution, mistakenly duplicated data, and irregularity of adverse event reporting.
Meet Our Cso

Dr. Zorayr Manukyan, PhD
Chief Scientific Officer
Dr. Zorayr Manukyan is the co-founder of ClinStatDevice, PCT Lab Research and ElmTreeResearch. Dr. Manukyan has strong expertise in designing, conducting, and performing the statistical analysis of all phases of clinical trials. Formerly, he was a Senior Director of Early Clinical Development at Pfizer, Inc. where he led multiple portfolios in rare diseases and immunoscience. Dr. Manukyan is active in clinical research with over three dozen publications in peer reviewed journals and has authored a patent for a risk-based monitoring technology – TrialSight. Dr. Manukyan earned a PhD in Statistical Sciences from George Mason University and a PhD in Biology from the National Academy of Sciences of Armenia. He also has an MS in Industrial Mathematics from the University of Kaiserslautern, Germany.
Dr. Manukyan is an Advisory Board Member of the Department of Statistics at George Mason University and author/co-author of 30+ publications in peer-reviewed journals.
Request a Demo
Feel in this form to request a Demo of our system. We will contact back and arrange a time that suits you.